I3M – CSIC – UPV

HORIZON-EIC-2023-TRANSITION-01 / 10113640

Truly portable MRI for extremity and brain imaging
anywhere & everywhere
Background
Mobile medical imaging devices are invaluable for clinical diagnostic purposes both inside and outside healthcare institutions. Unfortunately, Magnetic Resonance Imaging (MRI), the gold standard for numerous neurological and musculoskeletal conditions, is not readily portable. Recently, low-field MRI companies have demonstrated the first decisive steps towards portability within medical facilities and vehicles, but the scanners’ weight and dimensions are still incompatible with more demanding use cases such as in remote and developing regions, sports facilities and events, and medical or home healthcare.
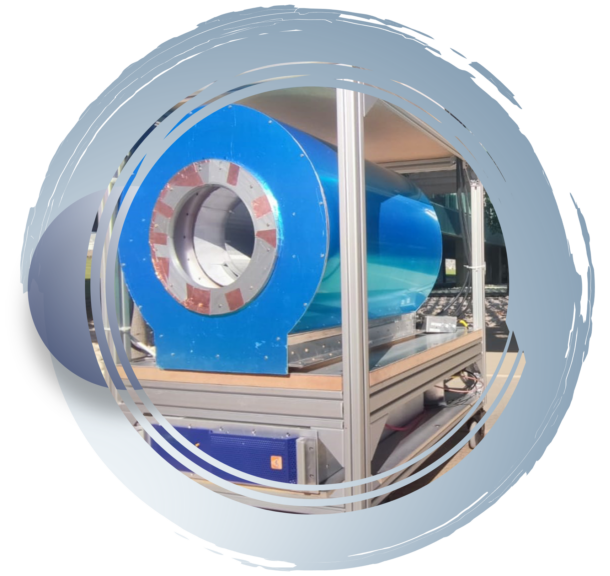
In a collaboration between CSIC, PHYS and LA FE, we have recently demonstrated in the Histo-MRI and PR Scanner projects in vivo images taken with a light, small footprint, low-field extremity MRI scanner outside the controlled settings provided by medical facilities, opening a path towards highly accessible MRI under circumstances previously unrealistic. To this end, we acquired images of a volunteer’s knee in indoor and outdoor environments, including the first-ever MRI images obtained at a patient’s home, and even in open air connected to a gasoline generator. In 2022, we installed the scanner in the medical facilities of the Ricardo Tormo Motor Racing Circuit during the four days of the Motorcycle Grand Prix held in Valencia (Spain), whose medical staff were able to visualize a number of lesions and conditions, including a traumatic arthritis which an X-ray radiograph and visual inspection had missed.
Goals
In this project we will take the necessary technical, industrial and entrepreneurial steps to tackle the massive deployment of low-field portable systems for point-of-care and bedside imaging in clinics large and small, home and hospice care, rural areas, sports facilities and events, making MRI available to a large fraction of the world population with no or insufficient access. To advance towards this highly ambitious goal, the objectives of the NextMRI project include
expand Technology
expanding the current technology to both extremity and brain imaging
Machine Learning
improving the diagnostic capabilities with machine learning
Improve portability
improving the portability and usability to meet end-user needs
Optimize costs
optimizing production costs
Clinical Trials
gathering medical evidence of the technology performance with clinical trials
Commercialisation
developing a sustainable business case and model towards commercialisation

State of Art
The most relevant milestone demonstrating the feasibility of NextMRI is the first-generation scanner. The resulting images show sufficient tissue contrast and spatial resolution to identify relevant anatomical features, including muscles, fat, cortical bone, bone marrow, tendons, ligaments, veins, arteries and fascia. Besides, we also show different contrast mechanisms, with weightings on T1, T2 and proton density. Professional radiologists at LA FE (Spain) confirm that the acquired images contain sufficient anatomical information to diagnose a large variety of articular diseases, including effusion, synovial engorgement, tendon disruption or bone fractures, within acceptable scan times. Furthermore, the portable scanner has been shown to operate successfully in three highly unconventional scenarios: an office room, in open air, and inside a patient’s living room. We have also acquired images from a volunteer diagnosed with lateral gonarthrosis due to cartilage damage in their right knee, and carrying a fixation metallic implant screwed to the femur. This appears sharp in our low-field acquisitions, but leads to high intensity artifacts around the metallic hardware in high-field MR images due to incorrect spin mapping. In 2022, we installed our scanner in the Motorcycle Grand Prix held in Valencia (Spain), where we scanned 14 subjects, running a total of 21 protocols for wrist, knee and ankle imaging. Out of eight scans on previously diagnosed lesions, only two (a meniscus tear and a Baker cyst) were not detected by the experts that evaluated our images. Notably, a subject reporting pain in a wrist revealed a traumatic arthritis which an X-ray radiograph and visual inspection had missed.
Methodology
Our ultimate goal is to site NextMRI scanners in clinics worldwide. We consider 3 phases:
Phase 1
(Nov 23 – Apr 25)
Prototype design and development, based on the Histo-MRI results and current advancements of the PR SCANNER project, and on the information gathered from clinicians and the relevant stakeholders
Phase 2
(Sep 25 – Sep 26)
Technical and usability validation of the system in lab and relevant environments
Phase 3
(until Dec 26)
Assess market acceptance solutions and develop the BP to reach new investors
The project will develop a Human Factors Plan, relying on User Centred Design (UCD). This approach involves end-users (different profiles of clinicians, management, patients, maintenance) and remaining stakeholders (institutions, purchase managers, etc.) in a Lean process during the entire product development. This process will follow the guidelines for CE marking, defined in the Medical Devices Regulation (MDR), to facilitate a future labelling.
Consortium
The team that composes the NextMRI consortium has been carefully designed to cover all the aspects that will arise in the project, with a balance between technical (CSIC, LUMC), clinical (LA FE, BERG) and business (PHYS) partners. Still, there is great interplay among partners in the majority of tasks. The involvement of the clinical partners goes beyond the mere trials, as both have extensive experience in research projects.

Funding








